 |
International School of Toulouse
Centre No. 1203 |
IB Biology |
SL / HL .. (circle one) |
| Expt No. 001 |
Title: Calculating Cell Size using a Microscope?
|
Time /h: 1 Hour |
| IB Syll Ref : 1.2 |
Student Name: ____________________________________ |
Date: _______ |
Assessment of ................. DCP: ______ (practice only) C &E: ______ MS: ______
Each student will need the following apparatus:
- a microscope with 4x, 10x and 40x objectives
- a small plastic ruler
- a cotton bud
- a dropping pipette
- fine tweezers
- two slides and coverslips
- live material of moss leaves, and /or onion cells
- access to methaline blue stain, iodine, a stage graticule and 500ml of steralising solution (for cheek cell & cotton bud disposal)
Aims are to learn how to:
- Measure the "field of view" diameter for x4, x10 and x40 objectives on a microscope
- Estimate the sizes of cells
- Record measurements to IB standards in a results table.
- Use Excel to plot bar graphs of the mean size with error bars showing the range of data, and the standard deviation.
- Use values of standard deviation to compare two samples of cell size.
- Calculate the T-test to compare differences between the two cell types.
- Plot a scatter graph of some sample data.
- Test for a correlation between the cell size and the size of the living thing.
Method
Measuring the "Field Of View" of your microscope.
- Set up a microscope on the x4 objective and focus it on the ruler scale.
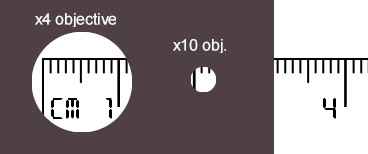
- Move the microscope stage until the 0.0mm of the ruler touches the left-most point of the field of view.
- Read of the diameter (in mm) and record your result neatly. (note how accurate you have been, e.g. +/- 0.5mm)
- Change the objective to x10 and repeat setps 2 & 3. The field of view should now be smaller (less than half the x4).
- Estimate the x40 objective's field of view by dividing the x4 diameter by 10. (use mm units).
- EXTENSION: check your measurements using an expensive microscope graticule.
Preparing slides of cells
- rub the inside of your cheek with a cotton bud for one minute
- put two drops of methaline blue stain on a clean slide
- rub the cheek cells from the cotton bud onto the methaline blue drops
- add a cover slip and clean off any excess stain with the corner of a paper towel.
- dispose of your contaminated cotton bud in the steralising solution.
(you must also put the slide into the steralising solution at end of the expt.)
Alternatively:
- peel a small piece of moss leaf, or the inner membrane of an onion layer, or a tree leaf
- mount it in a drop of iodine on a clean slide
- add a cover slip
Estimating the size of the cells

- Focus your microscope on the x4 objective onto your cells (ask the teacher if you are not sure)
- Once in focus turn the obectives to x10 and refocus USING FINE FOCUS.
- Identify one cell which is clearly visible.
- Estimate how many cells will fit into one diameter of your field of view.
- If more than 5 cells will fit then turn to the x40 objective and carefully re-focus as before and go back to step 3.
- Record this raw result YOURSELF in your own results table.
- Repeat steps 3, 4 and 6 for 20 cells.
- EXTENSION: Repeat the whole experiment with another biological specimen.
If time is running out, then you can swap results with another student. (You need results for two different cells)
Results
Record your measurements in Excel in a results table.
The Excel sheet might look like this:
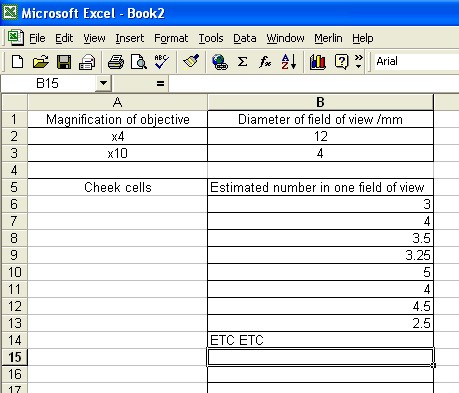
Ensure that your table is better than the one above;
- the "Estimated number" label doesn't say which field of view (x4, x40 or x10),
- the figures collected are not always to the same accuracy. "3" could be "3.0" or even "3.00"
- there needs to be a note about the recording error for the estimated number and the diameter.
(" +/- 1mm accuracy, +/-0.5mm at both ends of the ruler)
(the estimate was done by eye and could not be more accurate than +/- 0.25 cells, I estimated this error too.)
- the layout is also not perfect, all the numbers should be centered.
Data Processing
- Use Excel to calculate the mean of the data for two different types of cell.
(the formula for calculating a mean is =AVERAGE(range) )
Your improved results table could look something like this....
| Results of Experiment to estimate cell sizes of human cheek cells and onion cells |
| Magnification of objective |
Diameter of field of view
/mm |
|
|
| x4 |
12 |
|
|
| x10 |
4 |
|
|
|
|
|
|
Estimated number in one x10 field of view
(see footnote on errors) |
Estimated Size of Cheek Cells
/mm (+/- 0.25) |
Estimated number in one x10 field of view
(see footnote on errors) |
Estimated Size of Onion cells
/mm (+/- 0.25) |
| 3.00 |
1.33 |
4.00 |
1.00 |
| 4.00 |
1.00 |
3.00 |
1.33 |
| 3.50 |
1.14 |
4.00 |
1.00 |
| 3.25 |
1.23 |
3.00 |
1.33 |
| 5.00 |
0.80 |
2.75 |
1.45 |
| 4.00 |
1.00 |
3.00 |
1.33 |
| 4.50 |
0.89 |
3.50 |
1.14 |
| 2.50 |
1.60 |
3.00 |
1.33 |
| 3.00 |
1.33 |
4.00 |
1.00 |
| 4.25 |
0.94 |
3.75 |
1.07 |
| Calculations |
|
|
|
|
Cheek cells |
|
Onion cells |
| Mean Size /mm = |
1.13 |
|
1.20 |
- Use Excel to plot bar graphs with error bars showing the max and min values, called the "range of data",
The formula =MAX(range) returns the maximum value, and =MIN(range) returns the minium value.
To calculate the + or - error you need to subtract the average from the max or the min from the average.
|
Cheek cells |
|
Onion cells |
| Mean Size /mm = |
1.13 |
|
1.20 |
|
|
|
|
| = Max value - mean |
0.47 |
+ Yaxis error bar |
0.25 |
| = Mean - min value |
0.33 |
- Y axis error bar |
0.20 |
|
|
|
|
| Standard deviation |
0.247276198 |
|
0.175419152 |

when you add the error bars, you choose "custom" and then select the cells where you calculated the + or - error bar values.
- and error bars using the standard deviation. Excel formula is =STDEV(range)
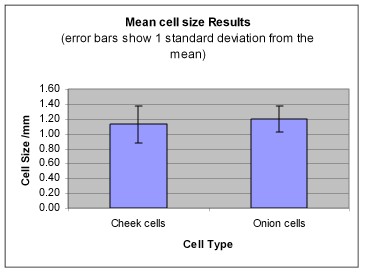
- Use the standard deviation to compare the two sets of data on cell sizes.
You may write something like this, if your graph looks like the one above...
"From the graph above the mean size of both cheek cells and onion cells is quite similar. The standard deviation of both sets of data is much greater then the difference between the two mean sizes, this suggests that there is no difference between the size of onion cells and cheek cells."
- Calculate the T-test to compare differences between the two cell types.
To be sure that a difference between two means is "not significant" you can test the probability that you got your two values when there really isn't any difference between the means. This test is called Students T-Test and you don't need to know how to calculate it.
You just need to know the Excel formula for an 'unpaired test' (Paired test are when you test something before and after an event, and we haven't done that in this lab) =TTESTU(range1,range2)
|
T-Test value of Probability of no difference = |
0.458064522 |
This is the formula used
=TTESTU(B7:B16,D7:D16) |
| P = 1.0 is certainty that there is no difference |
|
|
| P = 0.05 is the threshold value for a significant difference (at the 5% confidence level) |
The probability of these two samples of cell sizes being the same as each other is P=0.45
(45 times out of a 100 you could expect to get values like this when there is no difference between the cells)
From this T-Test it can be concluded that there is no significant difference between the size of onion cells and human cheek cells.
- Correlations
Last years class recorded these results in an investigation of cell sizes and organism sizes. All the values are in mm.
| Results of investigation into cell size |
| Organism |
Mean size of organism
/mm |
Mean cell size
/mm |
| Amoeba |
1.2 |
1.1 |
| Moss plant |
26.0 |
1.3 |
| Onion plant |
245.0 |
1.7 |
| frog |
165.0 |
1.1 |
| worm |
105.0 |
1.2 |
Plot a scatter graph of their data using Excel.
Install the User Defined Custom Chart Type from the Science Website. Your graph should look a bit like this:
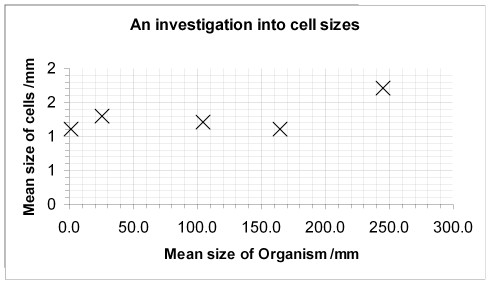
- EXTENSION work... to see if you really do have a correlation between two measurements it is possible to do a statistical test in 2 seconds.
You can calculate the R-squared value in three mouse clicks.
- Right click on a data point on your graph and click Add Trendline.
- Click the Options tab, and select Display R-squared value on chart.
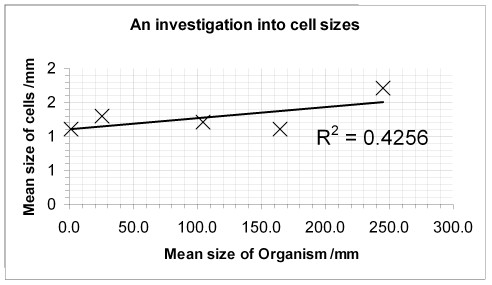
The R2 value of 0.4256 shows that 42% of the variation in the cell size correlates to the organism size.
It DOESN'T prove that big cells cause big organisms, nor that big organisms cause their cells to grow bigger.
It only shows that there is some link between the two variables.
There could be a third factor which causes both variables to incease. E.g. animals which have good nutrition could have bigger cells and grow bigger, because of their nutrition.
Evaluation ???






